 HUAWIN-深圳
HUAWIN-深圳
 +86 0755 28266382
+86 0755 28266382
 info@huawinlab.com
info@huawinlab.com
 深圳市宝安区洲石路743号深业U中心A栋7F
深圳市宝安区洲石路743号深业U中心A栋7F
 HUAWIN-东莞
HUAWIN-东莞
 +86 0769 85880813
+86 0769 85880813
 info@huawinlab.com
info@huawinlab.com
 深圳市宝安区洲石路743号深业U中心A栋7F
深圳市宝安区洲石路743号深业U中心A栋7F
2017年5月5日,欧盟通过其官方渠道Official Journal正式发布IVDR(REGULATION (EU) 2017/746)体外诊断设备新法规,将取代现行的IVD 98/79/EEC旧指令。由指令(Directive)升级为法规(Regulation),提高了文件的约束力;IVDR(EU) 2017/746是由欧盟委员会拟议并经欧洲议会和理事会认可的法律,将从根本上改变CE标志的机制和欧盟监管体外诊断设备的方式。IVDR(EU) 2017/746法规过渡期为5年,2022年5月4日起强制实行;包含了无特定医疗用途的产品,例如彩色隐形眼镜等。
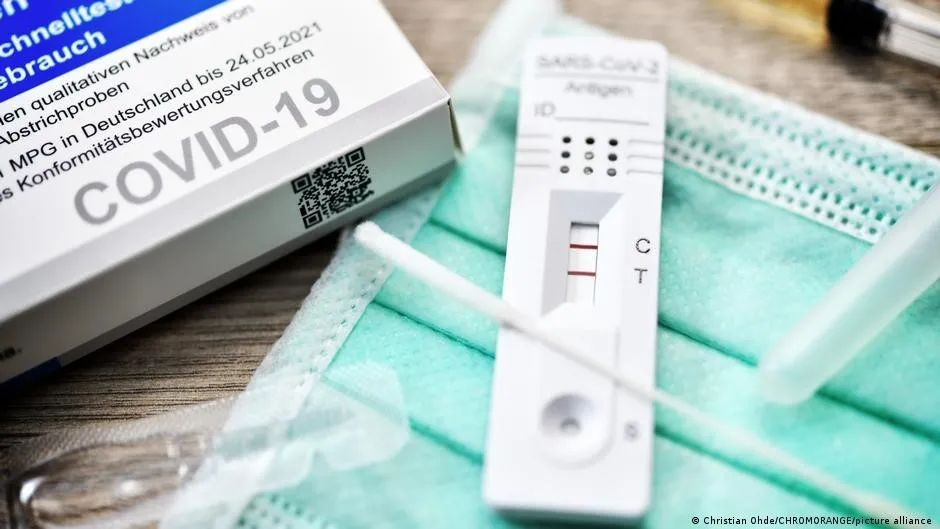
体外诊断设备的定义:
“体外诊断医疗设备”是指制造商预定用于体外检查从人体取得的样品,包括血液和组织供体的,无论单独使用或是组合使用的任何医疗器械,包括试剂,试剂产品,校准材料,控制材料,成套工具,仪表,装置,设备或系统,其唯一或主要目的是提供以下信息:
(a)关于生理学或病理学状态;或
(b)有关先性异常;或
(c)用于确定安全性以及与可能接受治疗者的相容性;或
(d)用于检查治疗措施
标本容器也应被视为体外诊断医疗器械;
体外诊断设备的分类:
体外诊断产品可分成5 类,List A、List B、自我检测器材(血糖检测除外)、其他类产品、性能评价器材,每一类的符合性评价途径(也就是获得CE认证的途径)各不相同。
指令相关的协调标准
|
EN 375 |
Information supplied by the manufacturer with in vitro
diagnostic reagents for professional use |
|
EN 376 |
Information supplied by the manufacturer with in vitro
diagnostic reagents for self-testing |
|
EN 591 |
Instructions for use in vitro diagnostic instruments
for professional use |
|
EN 592 |
Instructions for use for in vitro diagnostic
instruments for self-testing |
|
EN 928 |
In vitro diagnostic systems – Guidance on the
application of EN 29001 and EN 46001 and of EN 29002 and EN 46002 for
in vitro diagnostic medical devices |
|
EN 980 |
Graphical symbols for use in the labelling of medical
devices |
|
EN 1658 |
Requirements for marking of in vitro diagnostic
instruments |
|
EN 12286 |
In vitro diagnostic medical devices – Measurement of
quantities in samples of biological origin – Presentation of reference
measurement procedures |
|
EN 12287 |
In vitro diagnostic medical devices – Measurement of
quantities in samples of biological origin – Description of reference
materials |
|
EN 12322 |
In vitro diagnostic medical devices – Culture media for
microbiology – Performance criteria for culture media |
|
EN 13532 |
General requirements for in vitro diagnostic medical
devices for self-testing |
|
EN 13640 |
Stability testing of in vitro diagnostic medical
devices |
|
EN 13641 |
Elimination or reduction of risk of infection related
to in vitro diagnostic reagents |
|
EN 13975 |
Sampling procedures used for acceptance testing of in
vitro diagnostic medical devices - Statistical aspects |
|
EN ISO 14971 |
Medical devices – Application of risk management to
medical devices |
|
EN ISO 18153 |
In vitro diagnostic medical devices - Measurement of
quantities in biological samples - Metrological traceability of values
for catalytic concentration of enzymes assigned to calibrators and
control materials |
|
EN 61010-2-101 |
Safety requirements for electrical equipment for
measurement, control, and laboratory use - Part 2101: Particular
requirements for in vitro diagnostic (IVD) medical equipment Reference
document: IEC 61010-2-101 |
|
EN ISO 18153 |
In vitro diagnostic medical devices - Measurement of
quantities in biological samples – Metrological traceability of values
for catalytic concentration of enzymes assigned to calibrators and control materials |
|
EN 61010-2-101 |
Safety requirements for electrical equipment for
measurement, control, and laboratory use - Part 2- 101: Particular
requirements for in vitro diagnostic(IVD) medical equipment |